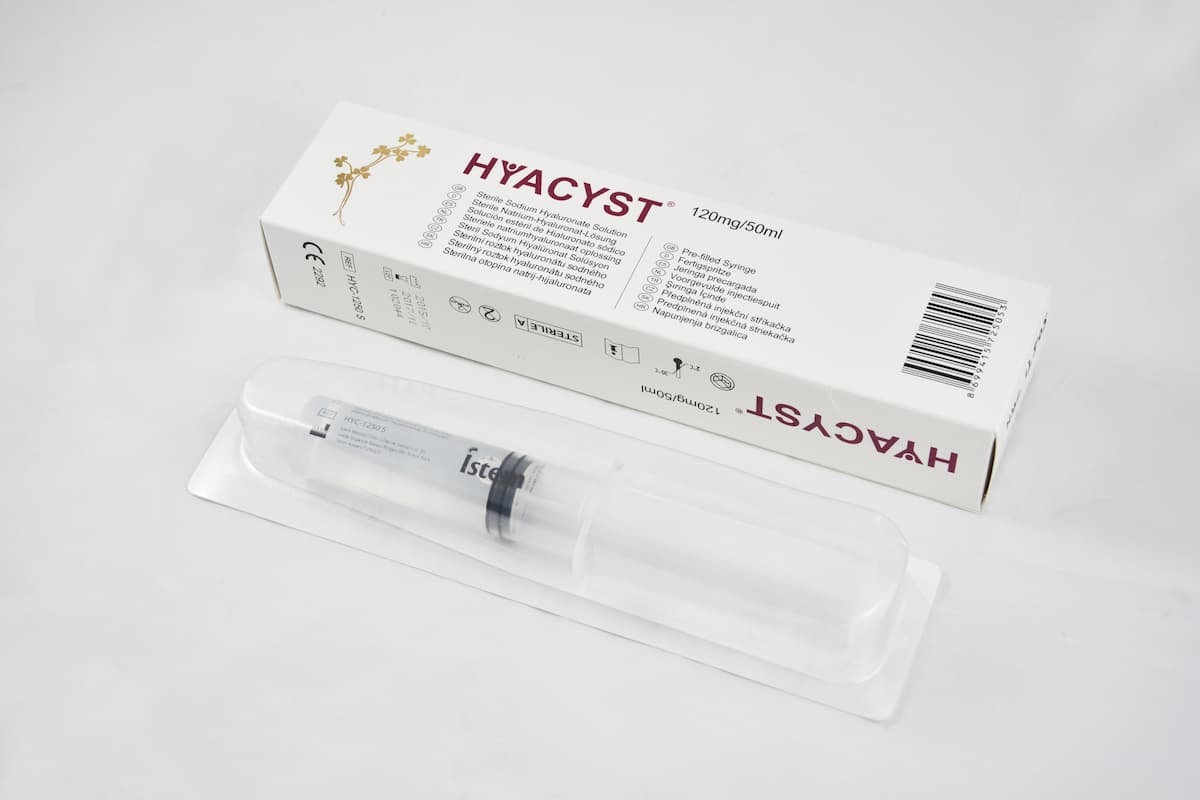
Hyacyst® (sodium hyaluronate) is a bladder instillation indicated for the temporary replenishment of the glycosaminoglycan (GAG) layer
Hyacyst® (sodium hyaluronate), Class IIa
Hyacyst® (sodium hyaluronate) is a bladder instillation indicated for the temporary replenishment of the glycosaminoglycan (GAG) layer.
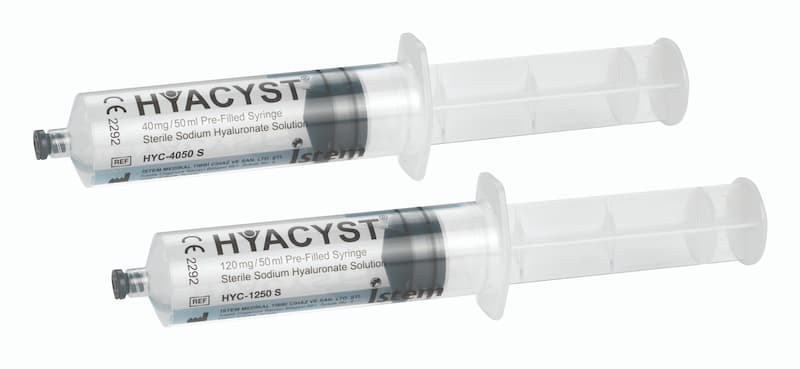
Hyacyst® (sodium hyaluronate) is available in strengths of 40mg/50ml and 120mg/50ml which are supplied as Pre-Filled Syringes (PFS).
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in the package leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine. Adverse events should also be reported to Syner-Med (Pharmaceutical Products) Limited. Tel. +44 020 8655 6380 (Option 1).